
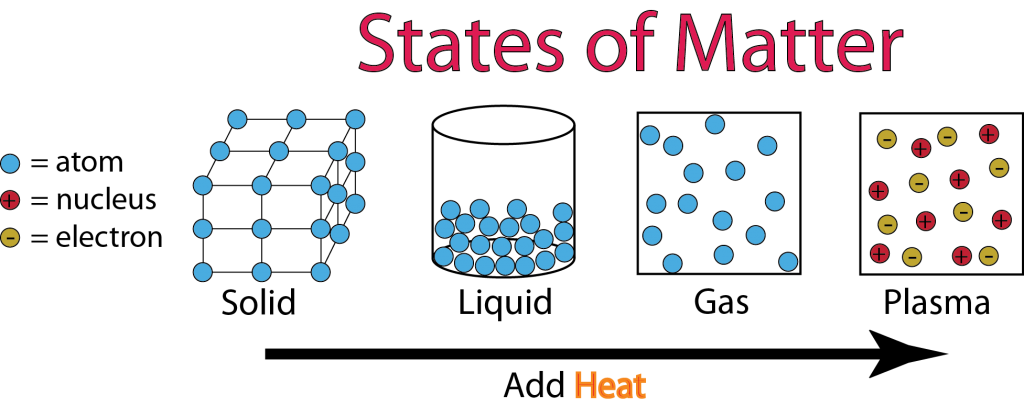
The quantum question: what constitutes a new state of matter? The exciton behaves a bit like a simple atom, while the dropleton is the first quasiparticle that has shown liquid-like behavior. The exciton and the dropleton form in similar ways, with electrons forming a bound state with a positively-charged “hole” where the electron should be. Some can be found in superconductors and are made-up of quasiparticles, a phenomenon that behaves like a particle without being one. Condensed matter physicsĪ field that has been particularly prolific in discovering new states is condensed matter physics, either solid or liquid. Beyond that state, there’s the quark-gluon plasma, when even the building blocks of particles are no longer constrained into tight configurations.
There, protons and electrons are so tightly packed together that they turn into neutrons due to beta decay. In these stars, electrons are in a degenerate gas form, which is a perfect heat conductor and behaves like a solid.Ĭontinuing to increase the pressure on matter, we reach neutron-degenerate matter, which is seen only in neutron stars. Increasing the pressure significantly, we get to the core of white dwarfs, which are likely made of electron-degenerate matter. On the other end of the scale at high temperatures, we start with supercritical fluids, when it is so hot and the pressure is high enough that it's impossible to distinguish if a fluid is a gas or a liquid. There are also Rydberg polarons, where it’s possible to have atoms inside other atoms. You can also have supersolids, those that move without friction, and superconductors, which are materials that have zero electrical resistance below a certain temperature. One of the most curious consequences of this state is that superfluids are capable of climbing out of the containers they are placed in. Staying at ultra-low temperatures, we can also experience superfluids – a second liquid state where the substance can flow without friction. Superfluids, supersolids, and superconductors At this limiting condition, quantum mechanic effects become dominant and we see peculiar states. It is obtained only in extremely low-density scenarios (one-hundred-thousandth the density of air) and ultra-low temperatures (a fraction of a degree above absolute zero). Under these conditions, the entire gas stops behaving like it is made of individual particles and instead behaves like a single macroscopic quantum system. The so-called fifth state of matter is the Bose-Einstein condensate, which happens only in a very dilute gas of particles known as bosons and when the temperature is close to absolute zero.

Pressure, heat, and cold can push substances into configurations with bizarre properties. To find and study them, however, we need to go to extremes. In the last century, scientists have come to realize that there are more states beyond the ones that we are (more or less) familiar with. It is the only metal we know of that is liquid at room temperature."For example, Ig Nobel laureates John Mainstone and Thomas Parnell performed a long-term experiment that measures the flow of a piece of pitch – bitumen – over many years, showing that even at room temperature bitumen flows and therefore belongs to the liquid state of matter.
STATE OF MATTER EXAMPLES FREE
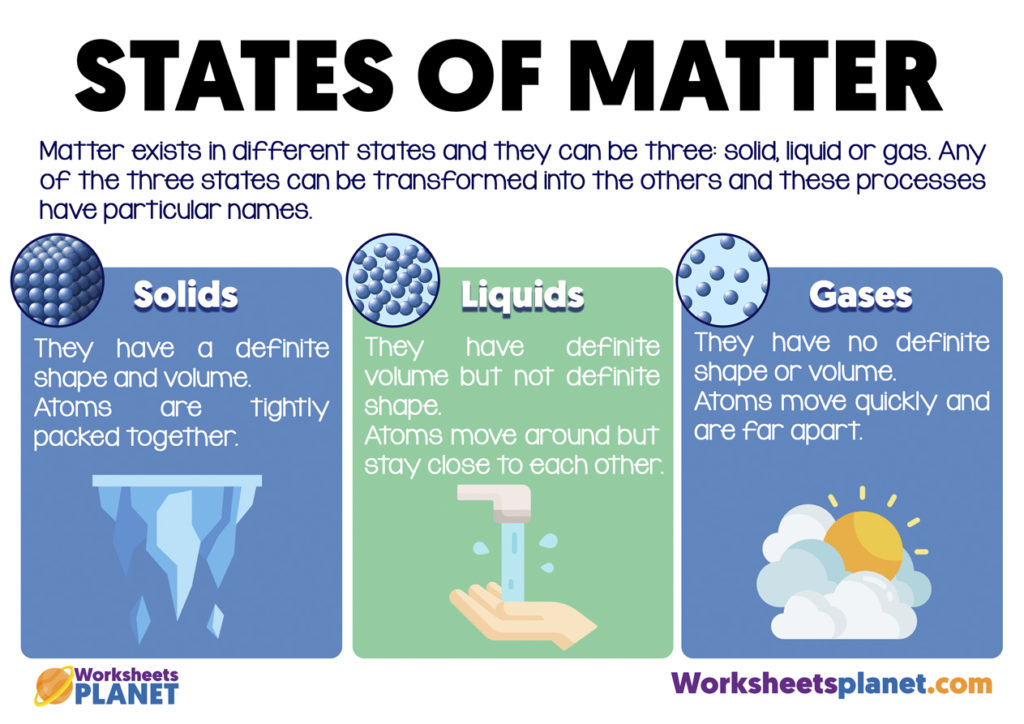
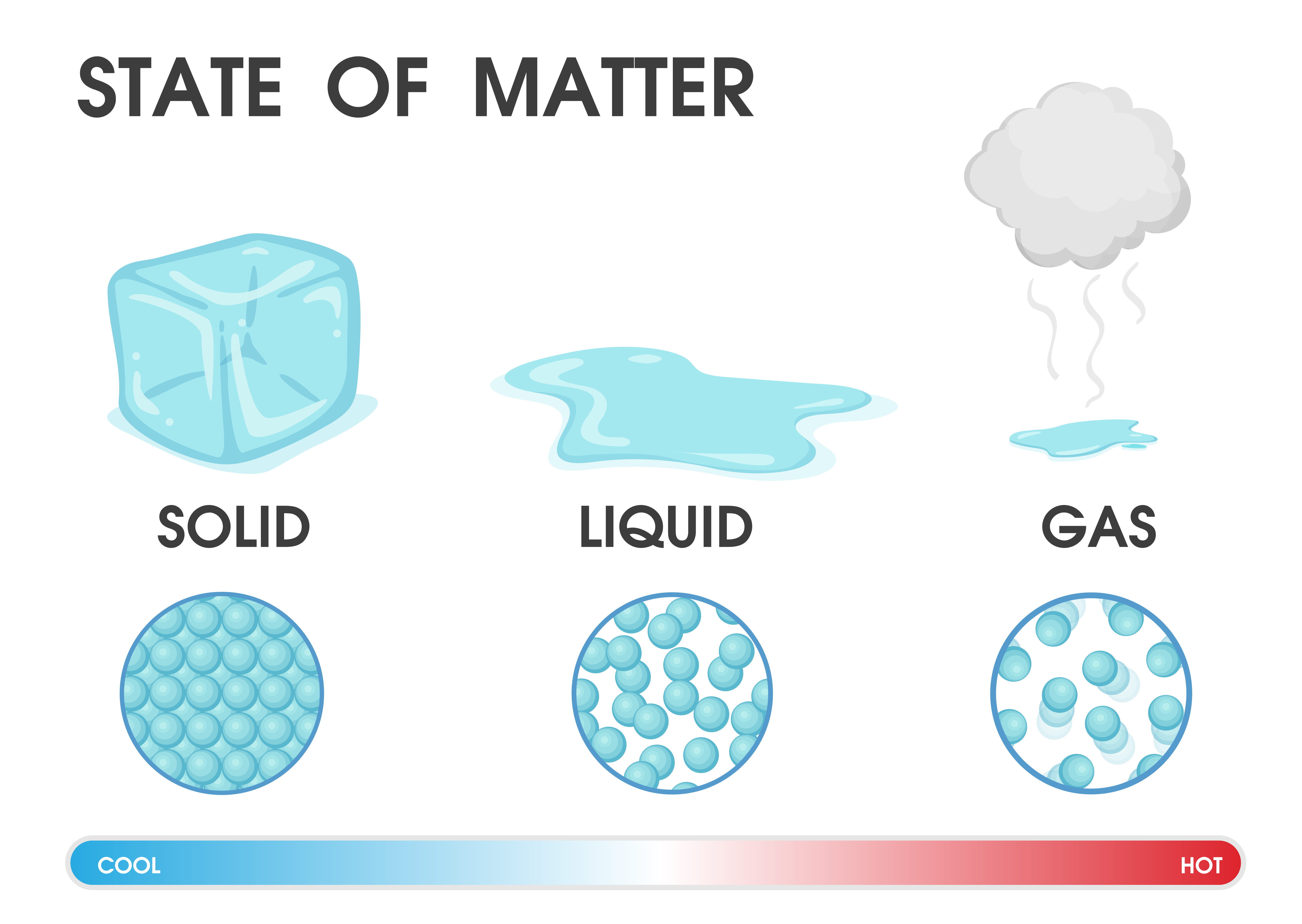
Particles are free to move over each other, but are still attracted to each otherĪ familiar liquid is mercury metal.No definite shape (takes the shape of its container).Liquids have the following characteristics: However, because the particles can move about each other rather freely, a liquid has no definite shape and takes a shape dictated by its container. In a liquid, the particles are still in close contact, so liquids have a definite volume. If the particles of a substance have enough energy to partially overcome intermolecular interactions, then the particles can move about each other while remaining in contact.
Images used with permission (public domain) Note that, as in the crystal, each Silicon atom is bonded to 4 oxygen atoms, where the fourth oxygen atom is obscured from view in this plane. (right) The random network structure of glassy \(SiO_2\) in two-dimensions. \): (left) The periodic crystalline lattice structure of quartz \(SiO_2\) in two-dimensions.


 0 kommentar(er)
0 kommentar(er)
